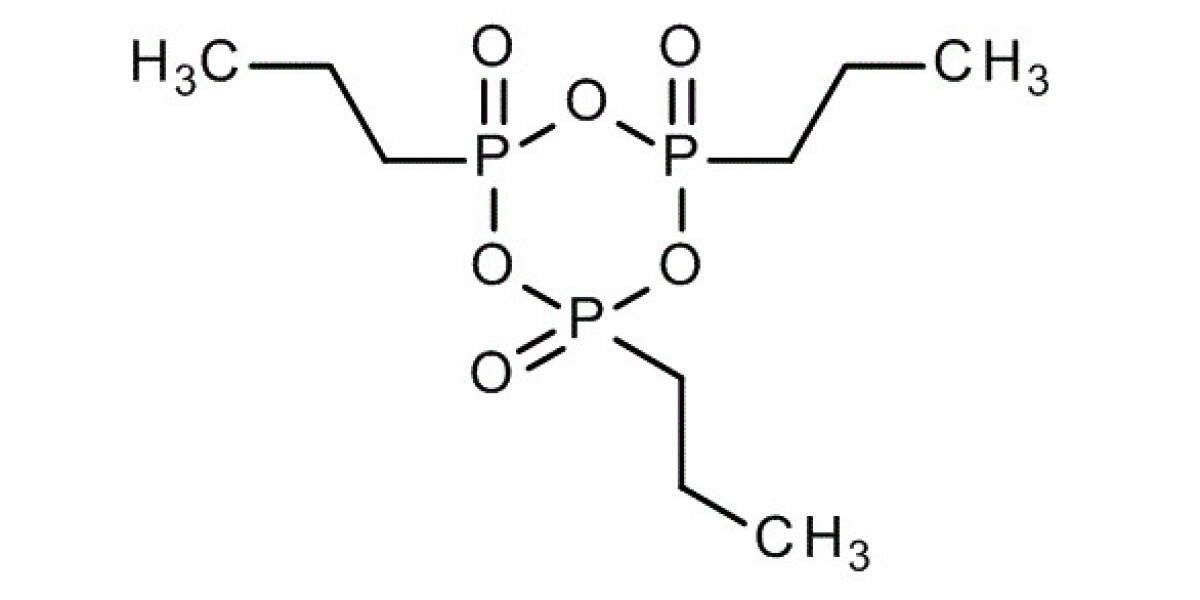
Propanephosphonic acid anhydride, commonly known as T3P, is a powerful reagent widely utilized in organic synthesis. This guide aims to provide a detailed overview of T3P, including its chemical properties, uses, working mechanism, handling precautions, and more.
What is Propanephosphonic Acid Anhydride (T3P)?
Propanephosphonic acid anhydride, abbreviated as T3P, is an efficient and versatile reagent used in peptide coupling, esterification, amidation, and various other organic transformations. It is valued for its high reactivity and low toxicity compared to other coupling agents.
Chemical Properties
- Chemical Formula: C3H9O6P
- Molecular Weight: 188.07 g/mol
- Appearance: Colorless to pale yellow liquid
- Solubility: Soluble in common organic solvents such as dichloromethane, tetrahydrofuran (THF), and dimethylformamide (DMF).
- Case no: 68957-94-8
Manufacturer
T3P is manufactured by several chemical companies that specialize in fine chemicals and reagents for laboratory and industrial use. These manufacturers adhere to stringent quality control measures to ensure the reagent's purity and effectiveness.
Releted Products:-
· Trimethylsilyl Trifluoromethanesulfonate
Uses of T3P
T3P is primarily used in:
- Peptide Synthesis: T3P is a preferred reagent for the formation of amide bonds, facilitating the synthesis of peptides and proteins.
- Esterification and Amidation: It is highly effective in converting carboxylic acids to esters and amides.
- Cyclization Reactions: T3P is used in various cyclization processes to form heterocyclic compounds.
- Dehydration Reactions: It acts as a dehydrating agent in the formation of anhydrides and other dehydrated products.
How T3P Works
T3P works by activating carboxyl groups (COOH) to form reactive intermediates that can then react with nucleophiles such as amines and alcohols. The process involves the following steps:
- Activation: T3P reacts with a carboxyl group to form an activated intermediate.
- Coupling: The activated intermediate reacts with a nucleophile (e.g., amine or alcohol) to form the desired product (e.g., amide or ester).
- Byproduct Formation: The reaction generates a water-soluble byproduct, propanephosphonic acid, which can be easily removed.
Handling and Storage
Proper handling and storage of T3P are crucial to maintain its stability and effectiveness:
- Storage: Store T3P in a cool, dry place, ideally at temperatures below 25°C. Keep it in a tightly sealed container away from moisture and light.
- Handling: Use appropriate personal protective equipment (PPE), such as gloves and safety goggles, when handling T3P. Work in a well-ventilated area or under a fume hood to avoid inhalation.
Precautions
When using T3P, consider the following safety precautions:
- Health Hazards: T3P can cause skin and eye irritation. Avoid direct contact and inhalation of vapors.
- Chemical Stability: Ensure T3P is stored properly to prevent hydrolysis and degradation.
- Environmental Safety: Dispose of T3P and its byproducts according to local regulations and guidelines for hazardous waste.
Side Effects and Toxicity
- Acute Exposure: May cause irritation to the skin, eyes, and respiratory system. In case of contact, rinse thoroughly with water and seek medical attention if necessary.
- Chronic Exposure: Prolonged exposure may lead to sensitization and allergic reactions in some individuals.
Applications in Research and Industry
- Pharmaceuticals: T3P is widely used in the synthesis of active pharmaceutical ingredients (APIs) and peptide drugs.
- Material Science: It is employed in the functionalization of materials and surfaces for improved properties and performance.
- Organic Synthesis: T3P is a valuable reagent in various organic transformations, including esterification, amidation, and cyclization reactions.
FAQ
1. Can T3P be used in aqueous solutions?
- T3P is generally used in organic solvents. It is not typically used in aqueous solutions due to its reactivity with water.
2. How should I dispose of T3P?
- Dispose of T3P according to local hazardous waste disposal regulations. Consult your institution's safety guidelines for proper disposal methods.
3. What is the shelf life of T3P?
- When stored properly, T3P has a shelf life of up to two years. Check the manufacturer's specifications for exact details.
4. Can T3P be used with other coupling reagents?
- T3P can be used in combination with other reagents to improve coupling efficiency and selectivity in certain reactions.
5. Is T3P compatible with all solvents?
- T3P is soluble in many organic solvents, including dichloromethane, THF, and DMF. However, it should be kept away from water and moisture to prevent degradation.
6. What should I do if T3P comes into contact with my skin?
- Rinse the affected area with plenty of water and seek medical attention if irritation persists.
Conclusion
Propanephosphonic acid anhydride (T3P) is an indispensable reagent in organic synthesis, offering high reactivity and low toxicity. Its ability to facilitate various chemical transformations, including peptide synthesis, esterification, and amidation, makes it a valuable tool for chemists and researchers. By understanding its properties, uses, and handling precautions, you can effectively and safely incorporate T3P into your laboratory practices.