Endoscopes Market
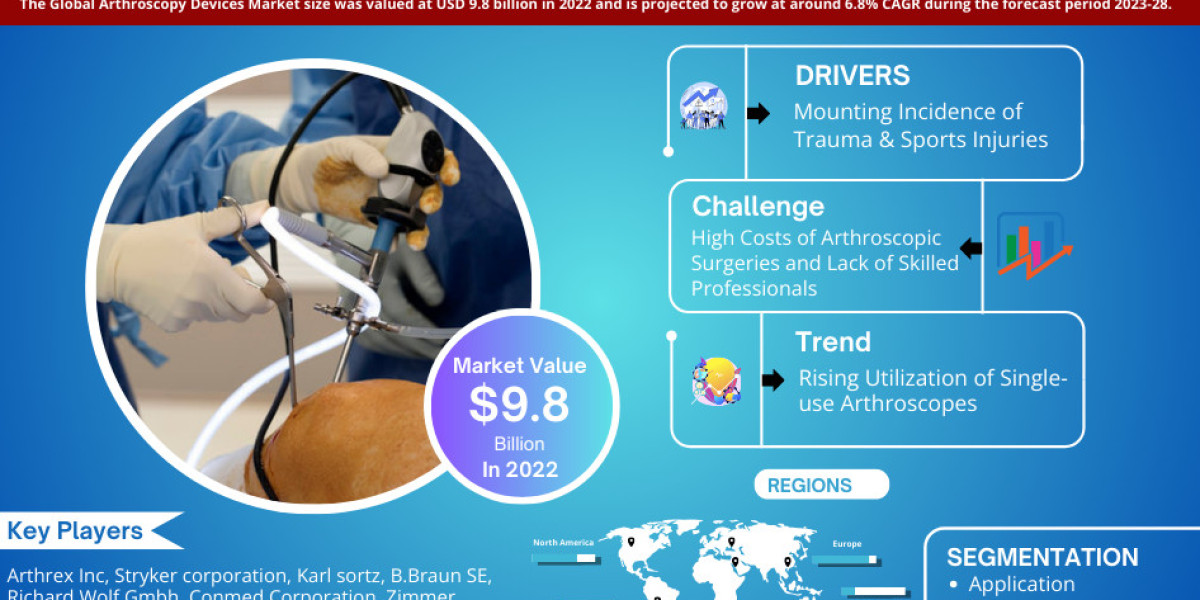
According to Straits Research, the global endoscopes market size was valued at USD 14.58 Billion in 2022. It is projected to reach from USD XX Billion in 2023 to USD 32.2 Billion by 2031, growing at a CAGR of 9.2% during the forecast period (2023–2031).
The Endoscopes Market has become a significant influence in contemporary medicine, enhancing efficiency, fostering innovation, and leading to better patient outcomes. This vibrant industry includes a wide variety of technologies, such as electronic health records (EHRs), telemedicine solutions, health information exchanges (HIEs), mobile health (mHealth) applications, and AI-powered tools. As the global healthcare environment faces issues like escalating costs, an aging population, and a growing need for personalized care, healthcare IT is crucial in tackling these challenges.
Get Free Sample Report PDF @ https://straitsresearch.com/report/endoscopes-market/request-sample
The uptake of healthcare IT solutions is driven by progress in cloud computing, big data analytics, and the Internet of Medical Things (IoMT). These innovations facilitate smooth data exchange, improve clinical decision-making, and encourage patients to participate more actively in managing their health. Additionally, incorporating IT into healthcare processes can optimize administrative workflows, minimize medical errors, and guarantee adherence to regulations.
The key players profiled in Endoscopes Market
- Olympus
- Karl Storz
- Ethicon U.S., LLC
- Pentax Medical
- Stryker
Regulatory Landscape in the Endoscopes Market
The Endoscopes Market functions within a complicated regulatory framework influenced by laws, standards, and guidelines aimed at guaranteeing patient safety, data protection, and service excellence. Although implementing IT solutions in healthcare offers potential for improved efficiency and innovation, managing the regulatory landscape poses considerable difficulties for stakeholders.
Regulatory Landscape
Health Information Privacy
The protection of patient data is paramount in healthcare IT, with regulations such as:- HIPAA (Health Insurance Portability and Accountability Act): In the U.S., HIPAA governs the use and sharing of protected health information (PHI). Compliance includes implementing safeguards for data confidentiality, integrity, and availability.
- GDPR (General Data Protection Regulation): In Europe, GDPR mandates strict controls over personal data, including health information, requiring informed consent and robust cybersecurity measures.
Interoperability Standards
Ensuring seamless data exchange between healthcare systems is critical for enhancing patient outcomes. Initiatives like the U.S. 21st Century Cures Act promote interoperability through standards like HL7 FHIR (Fast Healthcare Interoperability Resources).Device and Software Regulation
- FDA (Food and Drug Administration): In the U.S., healthcare IT devices and software, especially those classified as medical devices, are subject to FDA oversight. Software with diagnostic or treatment-related functions often falls under stringent regulatory scrutiny.
- MDR (Medical Device Regulation): In Europe, the MDR includes provisions for software as a medical device (SaMD), requiring conformity assessments and post-market surveillance.
Telehealth and Remote Care Regulations
The growth of telehealth services has resulted in changing regulations, including the temporary relaxations enacted during the COVID-19 pandemic, which eased licensure and reimbursement rules but underscored the necessity for standardized frameworks.
Get Detailed TOC @ https://straitsresearch.com/report/endoscopes-market/toc
Key Points from Table of Content
- Executive Summary
- Market Introduction
- Research Methodology
- Market Factor Analysis
- Market Dynamics
- Recent Trends Analysis
- Regulatory Landscape
- Segmentation
- Company Profile
Safety Concerns in the Endoscopes Market
Data Security and Breaches
The importance of cybersecurity continues to be a major issue since healthcare IT systems are major targets for cyber threats. Breaches of confidential patient information can lead to financial fines, harm to reputation, and disruption of care services.Software Errors and Reliability
Malfunctioning software or system outages can lead to severe repercussions, such as mistakes in diagnosis, postponed treatments, or negative incidents. It is crucial to prioritize thorough testing, validation, and continuous updates.Interoperability Challenges
Differences in standards among systems can result in miscommunication or discrepancies in data, which could jeopardize patient safety. Accurate real-time data exchange is essential for providing effective care.Ethical and Privacy Concerns
The growing implementation of AI and machine learning in healthcare brings forth ethical challenges, including biases in algorithms and the possible abuse of predictive analytics. Additionally, concerns regarding patients' consent and data ownership are crucial issues.Regulatory Non-compliance Risks
Failure to adhere to healthcare IT regulations can result in significant penalties, legal issues, and a deterioration of trust. It is essential for companies to remain alert to evolving regulatory demands in different areas.
Buy Endoscopes Market Research Report @ https://straitsresearch.com/buy-now/endoscopes-market
Region Included are
Global, North America, Europe, APAC, South America, Middle East & Africa, LATAM.
Country Level Break-Up: United States, Canada, Mexico, Brazil, Argentina, Colombia, Chile, South Africa, Nigeria, Tunisia, Morocco, Germany, United Kingdom (UK), the Netherlands, Spain, Italy, Belgium, Austria, Turkey, Russia, France, Poland, Israel, United Arab Emirates, Qatar, Saudi Arabia, China, Japan, Taiwan, South Korea, Singapore, India, Australia and New Zealand etc.
At long last, Endoscopes Market is an important source of direction for people and companies.
The market research report on the Global Endoscopes Market has been thoughtfully compiled by examining a range of factors that influence its growth, including environmental, economic, social, technological, and political conditions across different regions. A detailed analysis of data related to revenue, production, and manufacturers provides a comprehensive view of the global landscape of the Endoscopes Market. This information will be beneficial for both seasoned businesses and new entrants, assisting them in evaluating the investment prospects in this expanding market.
Thank you for taking the time to read this article; you can also obtain region-specific report versions, including Global, North America, Europe, APAC, South America, Middle East & Africa, and LAMEA) along with Forecasts for 2024-2032.
About US
Regardless of whether you're looking at business sectors in the next town or crosswise over continents, we understand the significance of being acquainted with what customers purchase. We overcome the issues of our customers by recognizing and deciphering just the target group, while simultaneously generating leads with the highest precision. We seek to collaborate with our customers to deliver a broad spectrum of results through a blend of market and business research approaches.
Contact US
Email ID : sales@straitsresearch.com
Phone No. : +1 646 905 0080 (U.S.) or +44 203 695 0070 (U.K.)