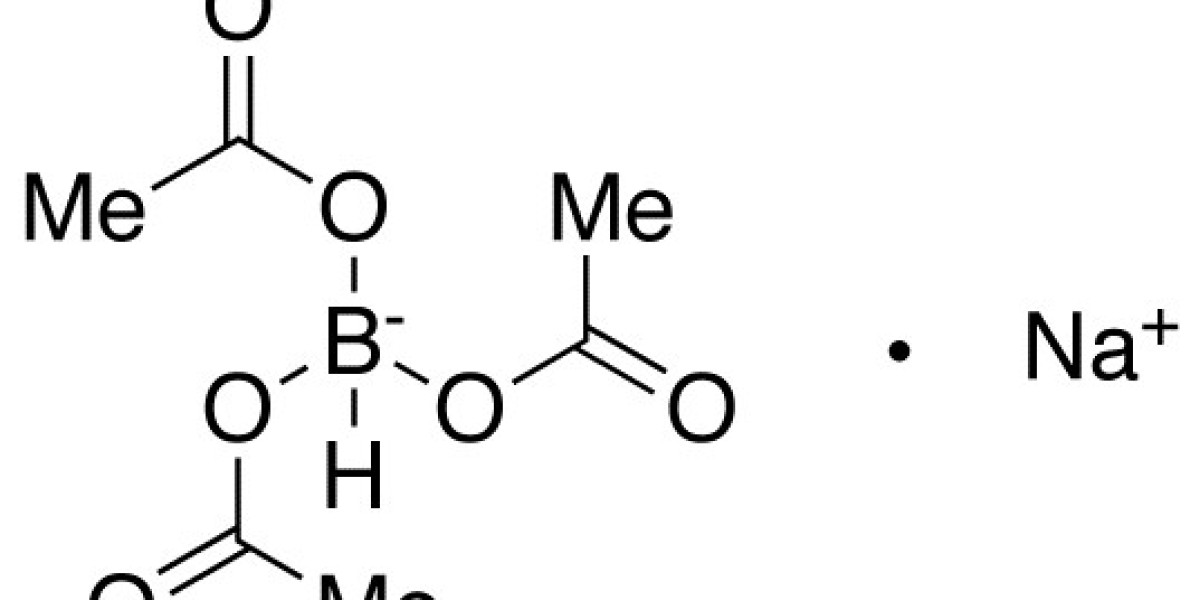
Sodium triacetoxyborohydride (STAB) is a widely used reducing agent in organic synthesis, particularly for reductive aminations. Its selective reactivity and ease of handling make it a go-to reagent for chemists working in both academic and industrial settings. This blog post will delve into its chemical properties, applications, and the best practices for its safe handling and use.
Chemical Properties
- Chemical Formula: C6H10BNaO6
- Molecular Weight: 211.94 g/mol
- CAS Number: 56553-60-7
- Physical State: Sodium triacetoxyborohydride is a white to off-white crystalline solid.
- Solubility: It is soluble in organic solvents such as acetonitrile, tetrahydrofuran (THF), and dichloromethane, but it is only sparingly soluble in water.
- Melting Point: Decomposes without a well-defined melting point.
- Density: Approx. 1.16 g/cm³
Key Features
- Selective Reducing Agent: Sodium triacetoxyborohydride is primarily used for reductive aminations, where it selectively reduces imines to amines while sparing other functional groups that are susceptible to reduction, such as esters, nitriles, and unsaturated ketones.
- Milder Alternative: Compared to other common hydride donors like sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4), STAB is milder, which helps in avoiding over-reduction or side reactions in complex molecules.
- Stability: It is more stable in both air and moisture compared to sodium cyanoborohydride (NaCNBH3), which is toxic and has similar applications. STAB's stability in moisture allows for easier handling under ambient conditions.
Common Applications
- Reductive Amination: This is by far the most prevalent use of sodium triacetoxyborohydride. In this process, an aldehyde or ketone reacts with a primary or secondary amine to form an imine or iminium intermediate, which is then reduced by STAB to yield a secondary or tertiary amine, respectively. It is highly favored for its selectivity and ability to tolerate various functional groups.
Example:

- Stereoselective Reactions: STAB can be used in stereoselective reductions, where it preferentially forms one stereoisomer over another, making it useful in the synthesis of chiral compounds.
- Mild Reductions: Beyond reductive aminations, it can also be used in the reduction of certain other functionalities like imines and enamines, but its reactivity is milder than stronger agents like lithium aluminum hydride.
Handling and Safety Considerations
- Hygroscopic: Sodium triacetoxyborohydride is mildly hygroscopic, so it is recommended to store it in a tightly sealed container away from moisture.
- Reaction with Water: Although more stable than sodium cyanoborohydride, STAB can slowly hydrolyze in water to form sodium borate and acetic acid. This can lead to exothermic reactions, so contact with water should be minimized.
- Toxicity and Irritancy: Like many borohydride compounds, STAB can be irritating to the skin and eyes. It should be handled with gloves, goggles, and protective clothing. It is also advisable to use it in a well-ventilated area or under a fume hood due to the possibility of acetic acid vapor formation during reactions.
Storage
- Dry Conditions: To maximize its shelf life, sodium triacetoxyborohydride should be stored in a cool, dry environment, ideally in an inert atmosphere (like nitrogen or argon) to prevent moisture uptake.
Advantages over Other Reducing Agents
- Sodium Borohydride: STAB is more selective than sodium borohydride, which tends to reduce a wider range of functional groups, leading to less controlled reactions.
- Lithium Aluminum Hydride: LiAlH4 is much more reactive and less selective, often leading to uncontrollable reactions in sensitive molecules. Additionally, it reacts violently with water, while STAB is easier and safer to handle.
- Sodium Cyanoborohydride: While both STAB and NaCNBH3 are used in reductive aminations, STAB is preferred due to its non-toxic nature and better stability under moist conditions.
Reactivity Tips
- Solvent Choice: Polar aprotic solvents like acetonitrile (MeCN), dichloromethane (DCM), and tetrahydrofuran (THF) are typically used with STAB to ensure optimal reduction.
- Temperature Control: Reactions with sodium triacetoxyborohydride are often carried out at room temperature, but lower temperatures can improve selectivity in some cases.
Limitations
- Hydrolysis Sensitivity: Despite its relative stability, STAB can still hydrolyze in water, so care must be taken when handling aqueous or wet reaction conditions.
- Reduction Scope: While excellent for reductive amination, STAB is less effective at reducing esters, nitriles, or heavily conjugated systems, which might require stronger reducing agents.
Conclusion
Sodium triacetoxyborohydride is an indispensable reagent in modern organic synthesis, particularly for its role in selective reductive amination. Its stability, ease of handling, and high selectivity make it a safer, more convenient alternative to other borohydride reducing agents. Whether you're working in medicinal chemistry, drug discovery, or complex molecule synthesis, this reagent is a reliable choice for achieving efficient reductions with minimal side reactions.